- Home
- About Us
- Services & Capabilities
- Production Systems
- Mammalian
$Our expertise spans discovery, development, and cGMP manufacture of biologics from mammalian cell culture. Leveraging 6 discovery platforms, a top-tier CMC development team, and an extensive supply chain, we deliver seamless programs for all your biologics drug development needs.
- Mammalian-Derived Product Services
$A true single-source provider from concept to commercialization for biologics produced from mammalian cell culture.
Mammalian-Derived Product Services
A true single-source provider from concept to commercialization for biologics produced from mammalian cell culture.
- Product types we support:
- Monoclonal Antibodies
$Discover information about the various drug development services we provide for this product type
Monoclonal Antibodies
Discover information about the various drug development services we provide for this product type
- Bispecific & Multispecific Antibodies
$Discover information about the various drug development services we provide for this product type
Bispecific & Multispecific Antibodies
Discover information about the various drug development services we provide for this product type
- Fc-Fusion Proteins
$Discover information about the various drug development services we provide for this product type
Fc-Fusion Proteins
Discover information about the various drug development services we provide for this product type
- Antibody Fragments
$Discover information about the various drug development services we provide for this product type
Antibody Fragments
Discover information about the various drug development services we provide for this product type
- Recombinant Proteins / Enzymes / Cytokines
$Discover information about the various drug development services we provide for this product type
Recombinant Proteins / Enzymes / Cytokines
Discover information about the various drug development services we provide for this product type
- Non-Vaccine Viral Products
$Discover information about the various drug development services we provide for this product type
Non-Vaccine Viral Products
Discover information about the various drug development services we provide for this product type
- Antibody Drug Conjugates (ADCs)
$Discover information about the various drug development services we provide for this product type
Antibody Drug Conjugates (ADCs)
Discover information about the various drug development services we provide for this product type
- Virus-Like Particles (VLPs)
$Discover information about the various drug development services we provide for this product type
Virus-Like Particles (VLPs)
Discover information about the various drug development services we provide for this product type
- Mammalian-Derived Product Services
- Microbial
$Comprehensive CMC development and cGMP manufacturing microbial fermentation platform. E. coli and yeast-based expression systems for the production of plasmid DNA and recombinant proteins.
- Microbial-Derived Product Services
$High quality, expert services for biologics produced from microbial fermentation.
Microbial-Derived Product Services
High quality, expert services for biologics produced from microbial fermentation.
- Product types we support
- Antibody Fragments
$Discover information about the various drug development services we provide for this product type
Antibody Fragments
Discover information about the various drug development services we provide for this product type
- Enzymes
$Discover information about the various drug development services we provide for this product type
Enzymes
Discover information about the various drug development services we provide for this product type
- Cytokines
$Discover information about the various drug development services we provide for this product type
Cytokines
Discover information about the various drug development services we provide for this product type
- Plasmid DNA (pDNA)
$Discover information about the various drug development services we provide for this product type
Plasmid DNA (pDNA)
Discover information about the various drug development services we provide for this product type
- Virus-Like Particles (VLPs)
$Discover information about the various drug development services we provide for this product type
Virus-Like Particles (VLPs)
Discover information about the various drug development services we provide for this product type
- RNA / mRNA
$Discover information about the various drug development services we provide for this product type
RNA / mRNA
Discover information about the various drug development services we provide for this product type
- Microbial-Derived Product Services
- Mammalian
- Capabilities
- Research
$Integrated Discovery Platform From Concept to IND
- Integrated Discovery Service
$Industry-leading expertise, state-of-the-art facilities, and multiple antibody generation technology platforms for the discovery of novel monoclonal, bispecific and multispecific antibodies, immunocytokines and other biologics.
Integrated Discovery Service
Industry-leading expertise, state-of-the-art facilities, and multiple antibody generation technology platforms for the discovery of novel monoclonal, bispecific and multispecific antibodies, immunocytokines and other biologics.
- Protein Sciences
$Specializing in monoclonal, bispecific, and multi-specific antibodies, fusion-proteins, cytokines, enzymes, and other biologics, we offer our customers high productivity and high quality antibody and protein production services for all of your research needs.
Protein Sciences
Specializing in monoclonal, bispecific, and multi-specific antibodies, fusion-proteins, cytokines, enzymes, and other biologics, we offer our customers high productivity and high quality antibody and protein production services for all of your research needs.
- Integrated Discovery Service
- Development
$Utilizing one of the world’s largest and most experienced development teams, we have the resources, technologies and expertise to drive your program toward IND and BLA in the most efficient and cost-effective manner.
- Protein Sciences
$We unite expert protein engineering capabilities with our high throughput and high productivity research-scale protein production services to enable all of your R&D initiatives.
Protein Sciences
We unite expert protein engineering capabilities with our high throughput and high productivity research-scale protein production services to enable all of your R&D initiatives.
- Cell Line Engineering
$Provided as either a standalone service offering or as part of our integrated CMC development platforms, WuXi Biologics provides extensive expertise and industry-leading timelines for cell line engineering and strain development across a wide range of biotherapeutics.
Cell Line Engineering
Provided as either a standalone service offering or as part of our integrated CMC development platforms, WuXi Biologics provides extensive expertise and industry-leading timelines for cell line engineering and strain development across a wide range of biotherapeutics.
- Analytical Sciences
$Our extensive analytical testing services offer top-tier expertise in method development for in-process, release, and stability assays, and support of cell line, process, and formulation development activities, product characterization, developability assessments and other key IND- and BLA-enabling studies.
Analytical Sciences
Our extensive analytical testing services offer top-tier expertise in method development for in-process, release, and stability assays, and support of cell line, process, and formulation development activities, product characterization, developability assessments and other key IND- and BLA-enabling studies.
- Process Development & Scale Up
$Multiple upstream and downstream PD labs facilitate the establishment and scale-up of fed-batch, intensified fed-batch, and continuous manufacturing approaches for diverse biotherapeutic modalities in both early and late-stage development.
Process Development & Scale Up
Multiple upstream and downstream PD labs facilitate the establishment and scale-up of fed-batch, intensified fed-batch, and continuous manufacturing approaches for diverse biotherapeutic modalities in both early and late-stage development.
- Late-Stage Development and Process Characterization
$We provide late-stage development and manufacturing services with advanced technology and a global network that ensures GMP manufacturing and rapid BLA filing.
Late-Stage Development and Process Characterization
We provide late-stage development and manufacturing services with advanced technology and a global network that ensures GMP manufacturing and rapid BLA filing.
- Formulation & Drug Product Development
$We provide advanced drug product development services spanning biologics, vaccines, and small molecules, with expertise in formulating clinical and commercial products in liquid, frozen, and lyophilized forms, compatible with various container closure systems such as vials, prefilled syringes, and other combination product types.
Formulation & Drug Product Development
We provide advanced drug product development services spanning biologics, vaccines, and small molecules, with expertise in formulating clinical and commercial products in liquid, frozen, and lyophilized forms, compatible with various container closure systems such as vials, prefilled syringes, and other combination product types.
- Cell Banking & Characterization
$Offering in-house, one-stop cell banking and cell line characterization services, compliant with global GMP regulations and ICH guidelines, we operate over 20 cGMP cell banking suites ensuring capacity availability and timely execution of this critical CMC development activity.
Cell Banking & Characterization
Offering in-house, one-stop cell banking and cell line characterization services, compliant with global GMP regulations and ICH guidelines, we operate over 20 cGMP cell banking suites ensuring capacity availability and timely execution of this critical CMC development activity.
- Viral Clearance
$Our extensive experience and expertise, along with EMA, ISO (CNAS), and CMA-certified laboratories, and exceptional track record for IND- and BLA-enabling viral clearance study acceptance by global regulatory agencies, form the cornerstones for this pivotal aspect of any CMC development program.
Viral Clearance
Our extensive experience and expertise, along with EMA, ISO (CNAS), and CMA-certified laboratories, and exceptional track record for IND- and BLA-enabling viral clearance study acceptance by global regulatory agencies, form the cornerstones for this pivotal aspect of any CMC development program.
- Regulatory Support
$Our experienced regulatory affairs team supports drug filing and registration with over 370 INDs/CTAs, 20+ BLAs/MAAs/NDAs/EUAs, and 150 Module 3 CMC packages since 2015.
Regulatory Support
Our experienced regulatory affairs team supports drug filing and registration with over 370 INDs/CTAs, 20+ BLAs/MAAs/NDAs/EUAs, and 150 Module 3 CMC packages since 2015.
- Protein Sciences
- Manufacturing
$Multiple state-of-the-art, premier quality cGMP clinical- and commercial-scale drug substance (DS) and drug product (DP) facilities across four countries for the production of a wide array of biologics from both mammalian and microbial expression systems.
- Clinical DS GMP Manufacturing
$Operating multiple high-quality, state-of-the-art clinical-scale cGMP facilities for biologics drug substance (DS) production utilizing both mammalian and microbial expression systems.
Clinical DS GMP Manufacturing
Operating multiple high-quality, state-of-the-art clinical-scale cGMP facilities for biologics drug substance (DS) production utilizing both mammalian and microbial expression systems.
- Clinical DP GMP Manufacturing
$Multiple, highly flexible clinical-scale drug product (DP) manufacturing facilities for the formulation, fill, labelling and packaging of biologics and parenterals under Current Good Manufacturing Practice (cGMP) conditions as defined by global regulatory agencies.
Clinical DP GMP Manufacturing
Multiple, highly flexible clinical-scale drug product (DP) manufacturing facilities for the formulation, fill, labelling and packaging of biologics and parenterals under Current Good Manufacturing Practice (cGMP) conditions as defined by global regulatory agencies.
- Commercial DS GMP Manufacturing
$We provide a global dual source manufacturing network that employs multiple large-scale drug substance (DS) GMP manufacturing facilities for the production of biologics utilizing our world-class operations and regulatory agency-approved quality systems.
Commercial DS GMP Manufacturing
We provide a global dual source manufacturing network that employs multiple large-scale drug substance (DS) GMP manufacturing facilities for the production of biologics utilizing our world-class operations and regulatory agency-approved quality systems.
- Commercial DP GMP Manufacturing
$Multiple large-scale drug product (DP) manufacturing facilities located across the globe for the high-quality formulation, fill, labelling and packaging of biologics, parenterals and placebo into a wide array of container closure systems.
Commercial DP GMP Manufacturing
Multiple large-scale drug product (DP) manufacturing facilities located across the globe for the high-quality formulation, fill, labelling and packaging of biologics, parenterals and placebo into a wide array of container closure systems.
- Clinical DS GMP Manufacturing
- Testing
$We provide extensive expertise for in-process, characterization, release and stability method development and testing either in support of our integrated biologics development platforms or as standalone projects. Our wide-range of analytical and biosafety testing Centers of Excellence and regulatory agency-approved quality control (QC) laboratories are the backbone of every service we provide our clients.
- Biosafety Testing
$High quality, in-house and EMA, ISO (CNAS) and CMA certified biosafety testing facilities for adventitious agent screening of raw materials, cell lines and unprocessed bulk along with our extensive viral clearance capabilities is available to provide our clients with a single-source biosafety testing service offering.
Biosafety Testing
High quality, in-house and EMA, ISO (CNAS) and CMA certified biosafety testing facilities for adventitious agent screening of raw materials, cell lines and unprocessed bulk along with our extensive viral clearance capabilities is available to provide our clients with a single-source biosafety testing service offering.
- Analytical Testing
$We offer an extensive array of assay development and analytical testing services, including bioassay development and forensic investigation analysis, along with other key characterization and IND and BLA-enabling studies throughout the various phases of drug development – all tailored for your unique product and project needs.
Analytical Testing
We offer an extensive array of assay development and analytical testing services, including bioassay development and forensic investigation analysis, along with other key characterization and IND and BLA-enabling studies throughout the various phases of drug development – all tailored for your unique product and project needs.
- Bioassay Method Development Services
- Product Characterization Service
- Routine Analytical Testing Services
- Centers of Excellence (CoE)
$Our CoEs support projects with specialized testing throughout the product lifecycle, expediting project timelines and ensuring analytical readiness for commercialization.
Centers of Excellence (CoE)
Our CoEs support projects with specialized testing throughout the product lifecycle, expediting project timelines and ensuring analytical readiness for commercialization.
- Global Quality Control (QC)
$Regulatory compliant, in-house, QC labs support all clinical and commercial GMP sites pre- and post-production, ensuring top-quality testing for products and oversight of other critical functions (e.g., environmental monitoring, cleaning validations, instrument life-cycle and sample/retain management and audits).
Global Quality Control (QC)
Regulatory compliant, in-house, QC labs support all clinical and commercial GMP sites pre- and post-production, ensuring top-quality testing for products and oversight of other critical functions (e.g., environmental monitoring, cleaning validations, instrument life-cycle and sample/retain management and audits).
- Biosafety Testing
- Research
- Quality
- Quality & Regulatory
$We provide world-class quality systems, harmonized across our global manufacturing sites and approved by multiple global regulatory agencies, including the U.S. FDA, EMA, NMPA, PMDA, MFDS, HSA, ANVISA and Health Canada for the production and testing of a wide array of biologics.
- Global Compliance
$Oversees global quality system and audit, IT quality, internal compliance, and risk analytics functions. This team embeds quality and compliance across the entire organization to ensure the products we manufacture are of the highest efficacy and safety standards and in line with your expectations.
Global Compliance
Oversees global quality system and audit, IT quality, internal compliance, and risk analytics functions. This team embeds quality and compliance across the entire organization to ensure the products we manufacture are of the highest efficacy and safety standards and in line with your expectations.
- Quality Assurance
$Global systems meet regulatory standards, ingrained commitment to quality from CEO to employees, harmonized QA across worldwide sites for biologics and vaccines production.
Quality Assurance
Global systems meet regulatory standards, ingrained commitment to quality from CEO to employees, harmonized QA across worldwide sites for biologics and vaccines production.
- Quality Control
$Regulatory compliant, in-house, QC labs support all clinical and commercial GMP sites pre- and post-production, ensuring top-quality testing for products and oversight of other critical functions (e.g., environmental monitoring, cleaning validations, instrument life-cycle and sample/retain management and audits).
Quality Control
Regulatory compliant, in-house, QC labs support all clinical and commercial GMP sites pre- and post-production, ensuring top-quality testing for products and oversight of other critical functions (e.g., environmental monitoring, cleaning validations, instrument life-cycle and sample/retain management and audits).
- Regulatory Affairs
$Our experienced regulatory affairs team supports our client’s CMC dossier, drug filing and registration needs. Since 2015 this team has supported 550+ INDs, CTAs, BLAs, MAAs, NDAs and EUAs, and written 200+ Module 3 CMC packages on behalf of our global clients.
Regulatory Affairs
Our experienced regulatory affairs team supports our client’s CMC dossier, drug filing and registration needs. Since 2015 this team has supported 550+ INDs, CTAs, BLAs, MAAs, NDAs and EUAs, and written 200+ Module 3 CMC packages on behalf of our global clients.
- Global Compliance
- Quality & Regulatory
- Featured Platforms
- Integrated Platforms
$Comprehensive, high-quality, end-to-end solutions driving efficiency, decreasing time to clinic and reducing project risks from concept to commercialization.
- Target to Lead
$Providing expertise, highly efficient tailored solutions, state-of-the-art technologies and world-class facilities for the engineering, generation, and optimization of antibodies and other biologics from target identification to final lead selection.
Target to Lead
Providing expertise, highly efficient tailored solutions, state-of-the-art technologies and world-class facilities for the engineering, generation, and optimization of antibodies and other biologics from target identification to final lead selection.
- DNA to IND
$Providing industry-leading 10-month (or less) DNA to IND timeline for biologics by leveraging our single-source, high-quality, and well-vetted development platform across all CMC activities.
DNA to IND
Providing industry-leading 10-month (or less) DNA to IND timeline for biologics by leveraging our single-source, high-quality, and well-vetted development platform across all CMC activities.
- IND to BLA
$Streamlined late-phase development and manufacturing services, ensuring BLA filing in 15 months or less, with global commercialization support through our highly-vetted development platforms.
IND to BLA
Streamlined late-phase development and manufacturing services, ensuring BLA filing in 15 months or less, with global commercialization support through our highly-vetted development platforms.
- Target to Lead
- Discovery Technologies
$A full spectrum of discovery services and technologies for the engineering, generation, characterization, optimization and selection of high-potency novel antibody therapeutics.
- Bispecific & Multispecific Antibodies – WuXiBody™
$The WuXiBody™ platform for the generation of bispecific (bsAb) and multispecific (msAb) antibodies speeds up drug development by 6-18 months, cuts production costs, and enables flexible assembly of mAb sequence pairs into various formats with different valency.
Bispecific & Multispecific Antibodies - WuXiBody™
The WuXiBody™ platform for the generation of bispecific (bsAb) and multispecific (msAb) antibodies speeds up drug development by 6-18 months, cuts production costs, and enables flexible assembly of mAb sequence pairs into various formats with different valency.
- Advanced Hybridoma Platform – WuXiHybrid™
$Using advanced technologies to generate diverse monoclonal antibodies (mAb) for various targets. Our experienced scientists overcome technical challenges to deliver high-quality mAbs to our clients worldwide.
Advanced Hybridoma Platform - WuXiHybrid™
Using advanced technologies to generate diverse monoclonal antibodies (mAb) for various targets. Our experienced scientists overcome technical challenges to deliver high-quality mAbs to our clients worldwide.
- Naive Phage Display Library – WuXiLiAb™
$Offering high-quality phage display library construction and customized library selection and screening services via our WuXiLiAb® human naïve / synthetic phage display libraries, VHH naïve / humanized VHH synthetic libraries and antibody immune libraries.
Naive Phage Display Library - WuXiLiAb™
Offering high-quality phage display library construction and customized library selection and screening services via our WuXiLiAb® human naïve / synthetic phage display libraries, VHH naïve / humanized VHH synthetic libraries and antibody immune libraries.
- VHH-based Multispecific Antibody Platform – SDARBody™
$Novel technology platform to enable the development of multispecific proteins. Highly flexible platform capable of generating multispecific antibody and protein therapeutics with high affinity, low immunogenicity risk and excellent developability characteristics.
VHH-based Multispecific Antibody Platform - SDARBody™
Novel technology platform to enable the development of multispecific proteins. Highly flexible platform capable of generating multispecific antibody and protein therapeutics with high affinity, low immunogenicity risk and excellent developability characteristics.
- ScFv-based BsAb & MsAb Antibody Platform – SKYBody™
$An innovative scFv (single-chain fragment variables)-based platform for the generation of bispecific or multispecific antibodies from nearly any sequence pair. The platform significantly improves scFv stability providing bsAbs and msAbs with strong developability characteristics.
ScFv-based BsAb & MsAb Antibody Platform - SKYBody™
An innovative scFv (single-chain fragment variables)-based platform for the generation of bispecific or multispecific antibodies from nearly any sequence pair. The platform significantly improves scFv stability providing bsAbs and msAbs with strong developability characteristics.
- VAST-B Single B-Cell Antibody Discovery Platform
$An advanced, high speed, high throughput and high resolution antibody screening strategy using Beacon® technology for the rapid discovery of monoclonal antibodies (mAb) from any host species.
VAST-B Single B-Cell Antibody Discovery Platform
An advanced, high speed, high throughput and high resolution antibody screening strategy using Beacon® technology for the rapid discovery of monoclonal antibodies (mAb) from any host species.
- Bispecific & Multispecific Antibodies – WuXiBody™
- Development Technologies
$Cutting-edge bioprocessing platforms and technologies designed to enable high-quality biologics to enter clinical trials faster, more efficiently and more cost-effectively.
- Ultra-Intensified Fed-batch platform – WuXiUI™
$Ultra-intensified fed-batch bioprocessing platform utilizes an intermittent perfusion approach to achieve 3-6-fold higher productivity and 60 – 80% reduction in manufacturing cost of goods compared to traditional fed-batch processes.
Ultra-Intensified Fed-batch platform - WuXiUI™
Ultra-intensified fed-batch bioprocessing platform utilizes an intermittent perfusion approach to achieve 3-6-fold higher productivity and 60 – 80% reduction in manufacturing cost of goods compared to traditional fed-batch processes.
- Rapid Research Material Generation – WuXian™
$Custom protein generation services, producing high-quality, research-grade proteins/antibodies by using our industry-leading team of cell culture, purification and analytical experts and high-throughput, cost-effective production technologies.
Rapid Research Material Generation - WuXian™
Custom protein generation services, producing high-quality, research-grade proteins/antibodies by using our industry-leading team of cell culture, purification and analytical experts and high-throughput, cost-effective production technologies.
- Cell Line Development – WuXia™
$Proven, high-yielding (up to 11 g/L) and high-quality cell line development platform, accepted by regulatory agencies worldwide.
Cell Line Development - WuXia™
Proven, high-yielding (up to 11 g/L) and high-quality cell line development platform, accepted by regulatory agencies worldwide.
- Continuous Bioprocessing – WuXiUP™
$This ultra-high productivity continuous bioprocessing platform, achieves 5-15X greater productivity, delivering high-yield, quality drug products with flexibility and cost-effectiveness through intensified perfusion culture, continuous harvest and optional downstream continuous product capture step.
Continuous Bioprocessing - WuXiUP™
This ultra-high productivity continuous bioprocessing platform, achieves 5-15X greater productivity, delivering high-yield, quality drug products with flexibility and cost-effectiveness through intensified perfusion culture, continuous harvest and optional downstream continuous product capture step.
- ADC Development Platform – WuXiDAR4™
$Our drug-to-antibody ratio and enrichment technology greatly enhances DAR4 (four payload molecules per mAb) percentage in the final ADC product and improves conjugation efficiency.
ADC Development Platform - WuXiDAR4™
Our drug-to-antibody ratio and enrichment technology greatly enhances DAR4 (four payload molecules per mAb) percentage in the final ADC product and improves conjugation efficiency.
- High Concentration & HTP DP Platform – WuXiHigh™
$Advanced high throughput formulation platform for development of high concentration biologics drug products using distinctive viscosity reducers and synergistic excipient combinations to enhance formulation stability and reduce viscosity.
High Concentration & HTP DP Platform - WuXiHigh™
Advanced high throughput formulation platform for development of high concentration biologics drug products using distinctive viscosity reducers and synergistic excipient combinations to enhance formulation stability and reduce viscosity.
- Ultra-Intensified Fed-batch platform – WuXiUI™
- Manufacturing Technologies
$Advanced biomanufacturing platforms empower our global healthcare partners to deliver biologics to the clinic and on to the market for the benefit of patients worldwide.
- Single-Use Bioreactors
$We operate several of the world’s largest single-use bioreactor facilities for biologics manufacturing, leveraging the risk reduction, flexibility, cost-effectiveness, and eco-friendliness offered by these systems.
Single-Use Bioreactors
We operate several of the world’s largest single-use bioreactor facilities for biologics manufacturing, leveraging the risk reduction, flexibility, cost-effectiveness, and eco-friendliness offered by these systems.
- Scale-Out Biomanufacturing
$Helping to pioneer this biomanufacturing strategy for higher volume production, we can help you reduce risk and increase manufacturing flexibility across the entire product lifecycle by using multiple, same-scale bioreactors to meet clinical and market demand.
Scale-Out Biomanufacturing
Helping to pioneer this biomanufacturing strategy for higher volume production, we can help you reduce risk and increase manufacturing flexibility across the entire product lifecycle by using multiple, same-scale bioreactors to meet clinical and market demand.
- Robotic Aseptic Filling
$Reducing manufacturing risk through the use of this fully programmable, robotic, gloveless, isolator-based technology for advanced aseptic drug product fill processing. These systems provide extensive flexibility to support complex fills across a wide range of biologics and container closure systems.
Robotic Aseptic Filling
Reducing manufacturing risk through the use of this fully programmable, robotic, gloveless, isolator-based technology for advanced aseptic drug product fill processing. These systems provide extensive flexibility to support complex fills across a wide range of biologics and container closure systems.
- Continuous Manufacturing Process
$Combining intensified perfusion culture (IPC) and continuous direct product capture (CDPC), and advanced equipment for increased productivity and cost reduction.
Continuous Manufacturing Process
Combining intensified perfusion culture (IPC) and continuous direct product capture (CDPC), and advanced equipment for increased productivity and cost reduction.
- Single-Use Bioreactors
- Animal Health Technologies
$One-stop solution for superior animal health outcomes.
- Animal Health Technologies
$We offer CMC-compliant, one-stop solution for animal health, to support taking drug substance and drug product from research to manufacturing.
Animal Health Technologies
We offer CMC-compliant, one-stop solution for animal health, to support taking drug substance and drug product from research to manufacturing.
- Animal Health Technologies
- Integrated Platforms
- Production Systems
- Sustainability
- Careers
- Investors
- News & Resources
- Contact Us
- 中文 / CN
- Deutsch / DE
- 日本語 / JP
- 한글 / KR
WuXi Biologics
Offering End-to-End Solutions
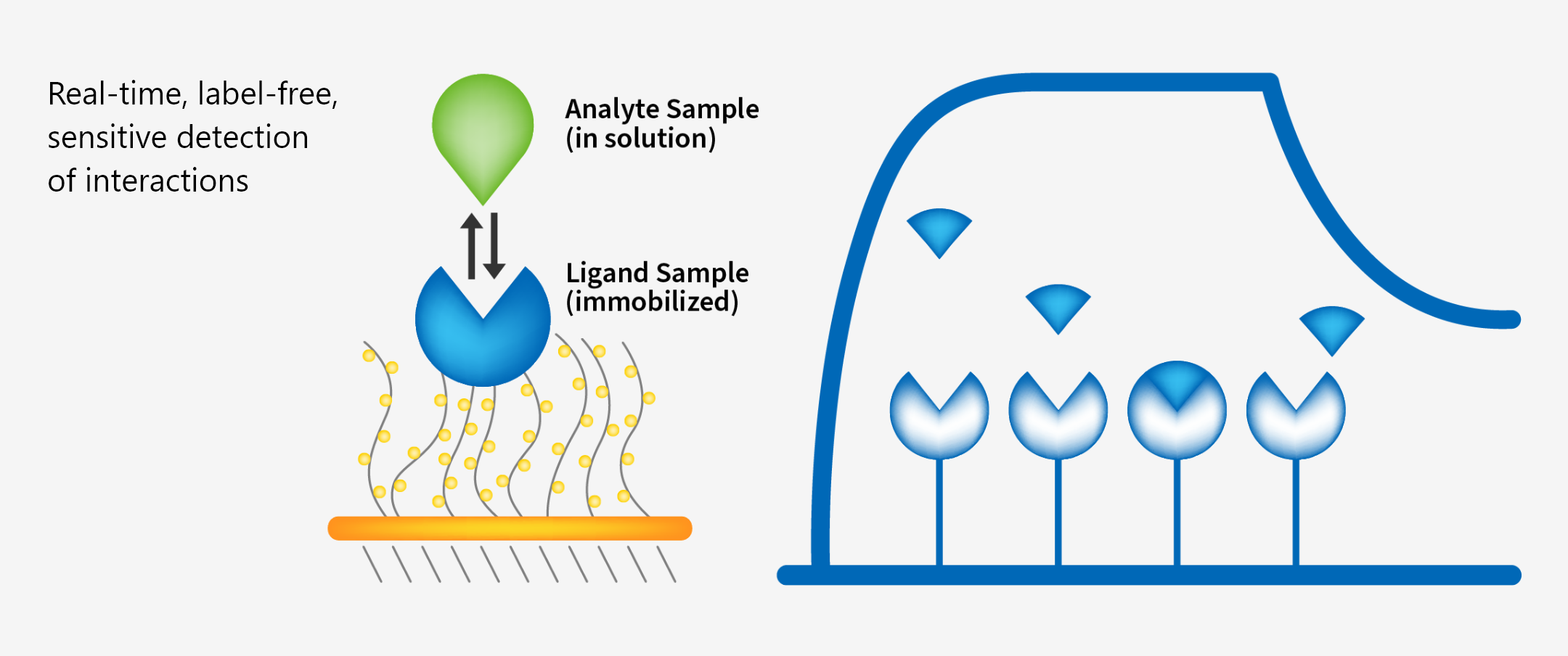
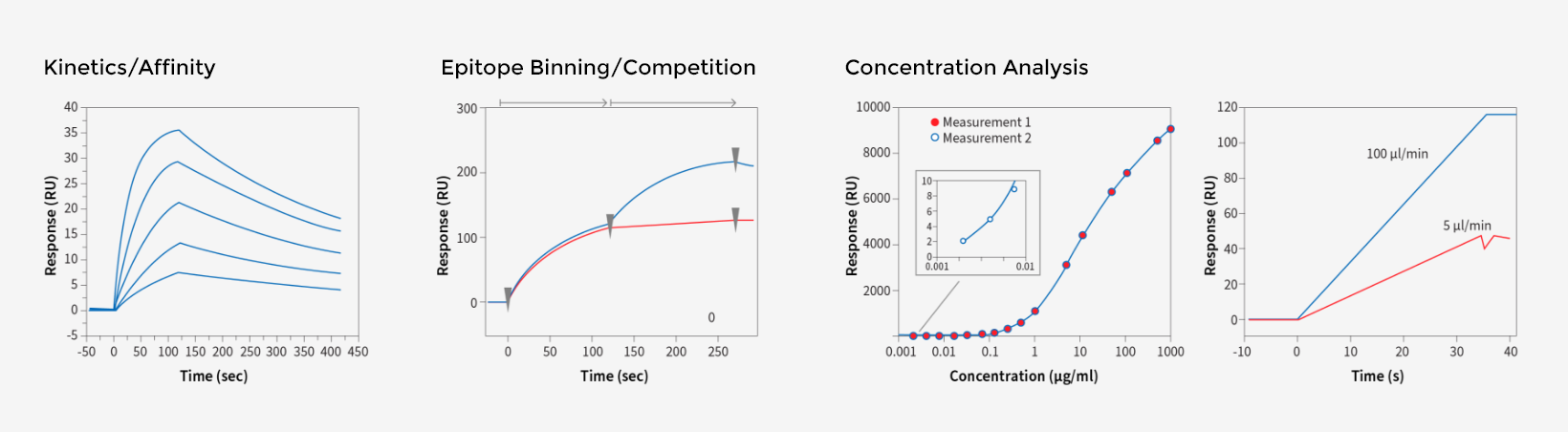
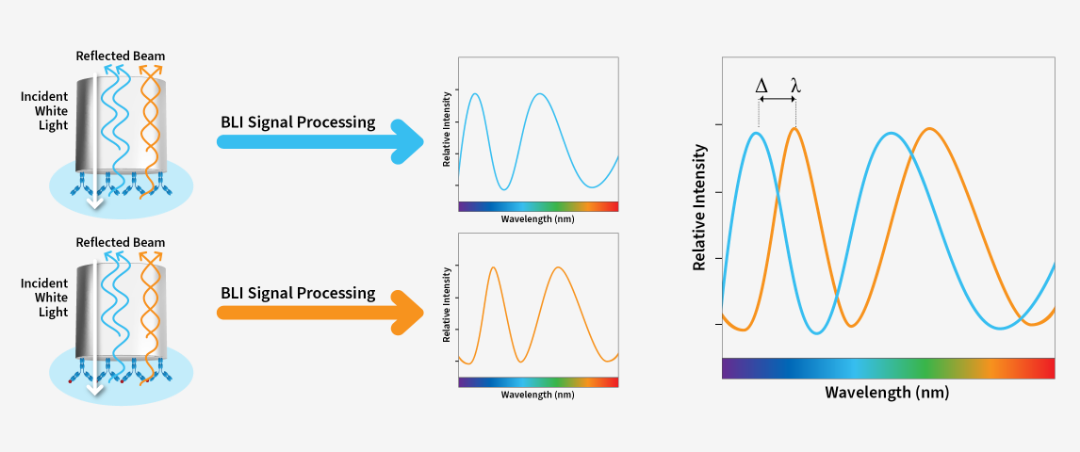
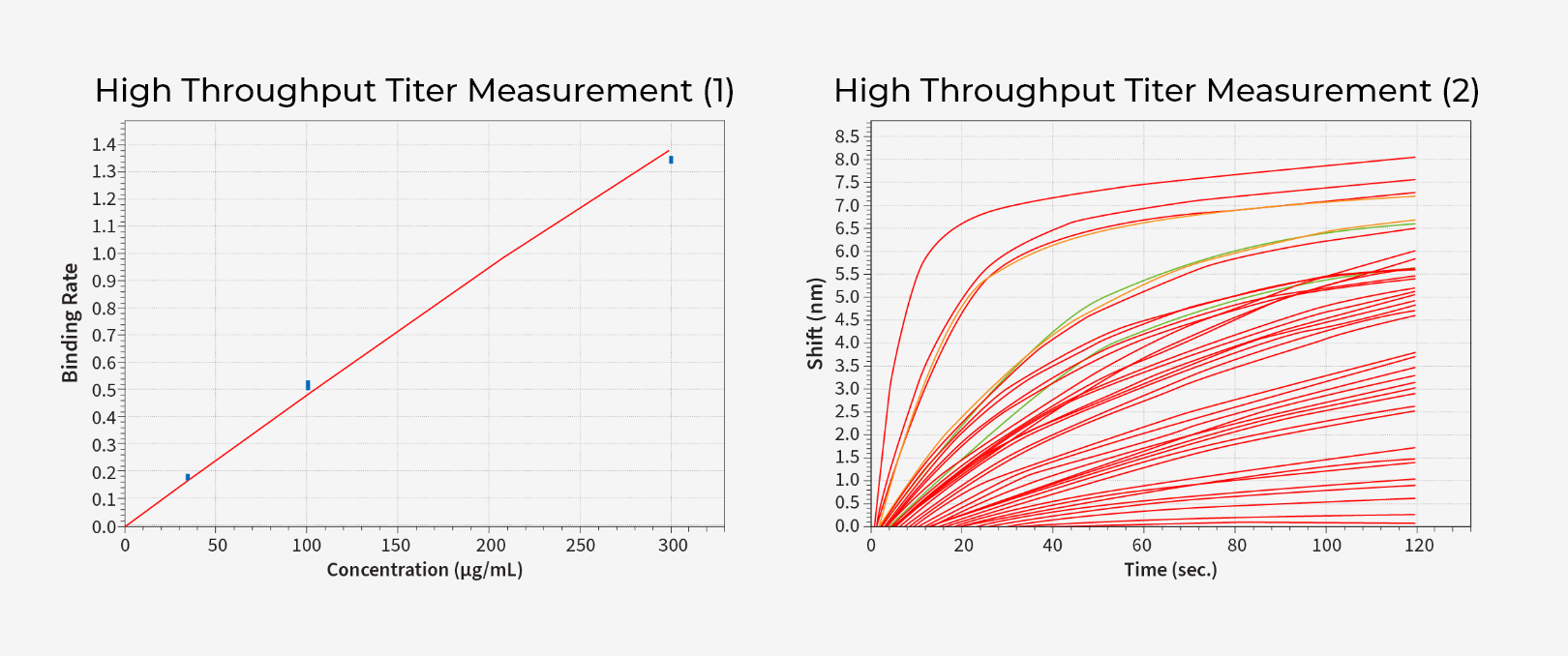
WuXi Biologics provides comprehensive testing for all aspects of your lead molecule to help you assess their molecules early on. From purity/impurity testing, structure characterization including mass spectrometry based protein ID and post translational modification analysis, to SPR/ELISA/cell-based activity assay, our protein analytics platform is there to help you triage/optimize your candidates efficiently and effectively as possible.
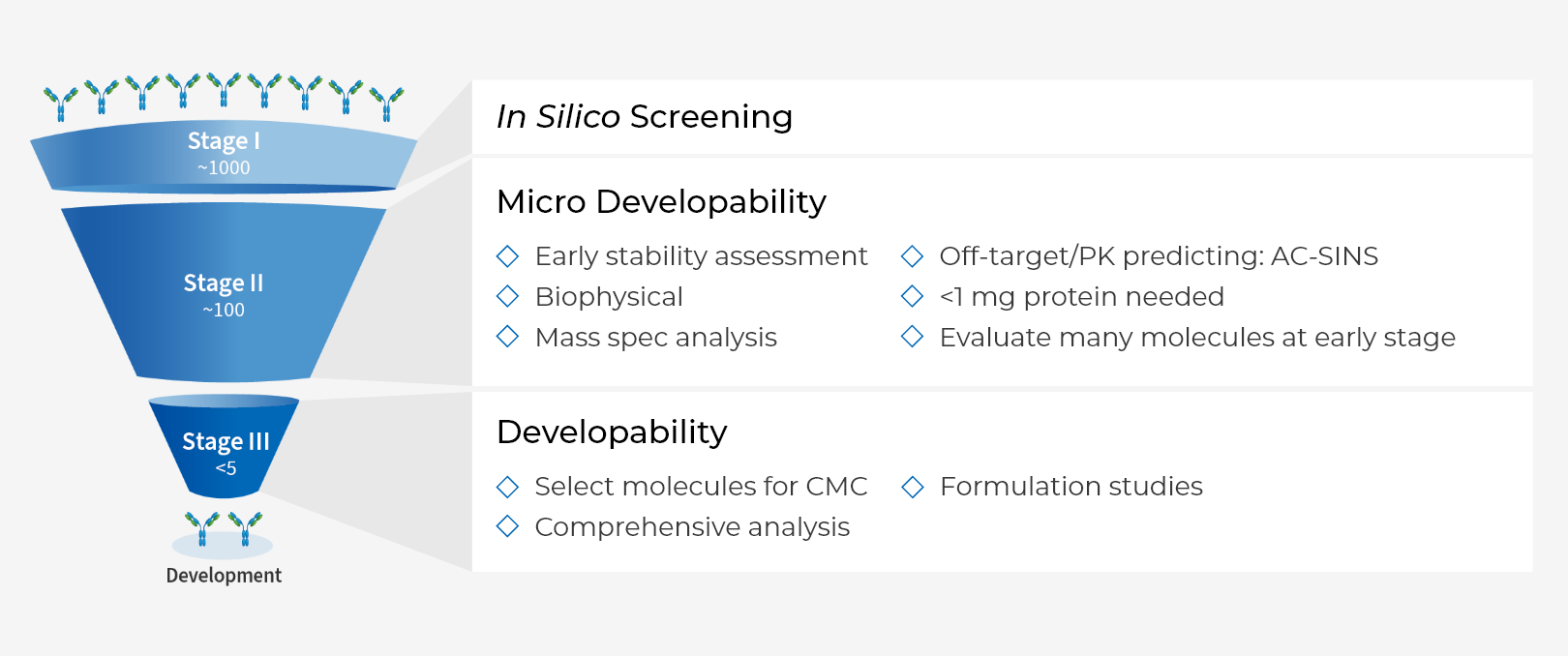
Micro Developability
- High throughput, low concentration and purity requirement
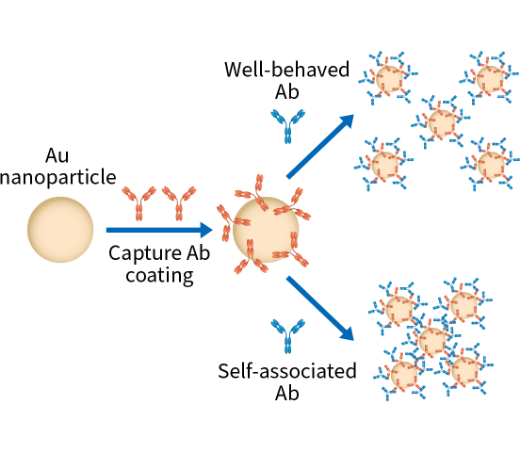
- Assessment of antibody’s propensity of self-association/ aggregation, viscosity etc.
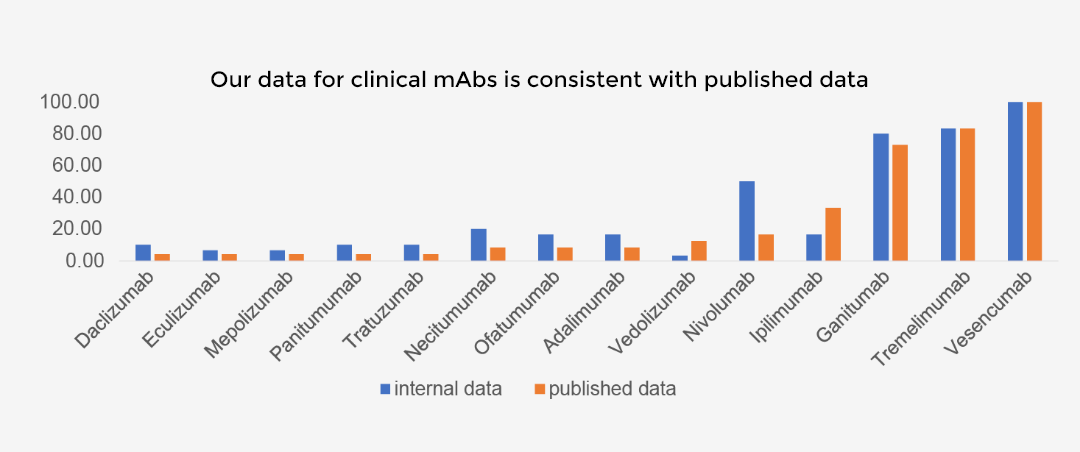
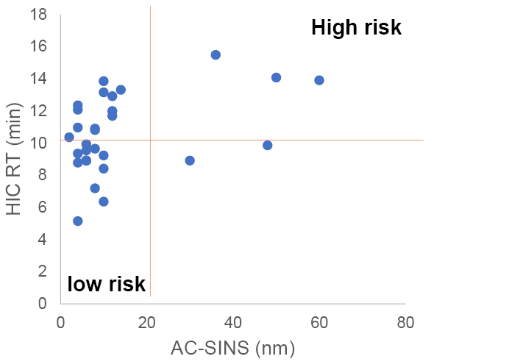
AC-SINS HIC Correlation
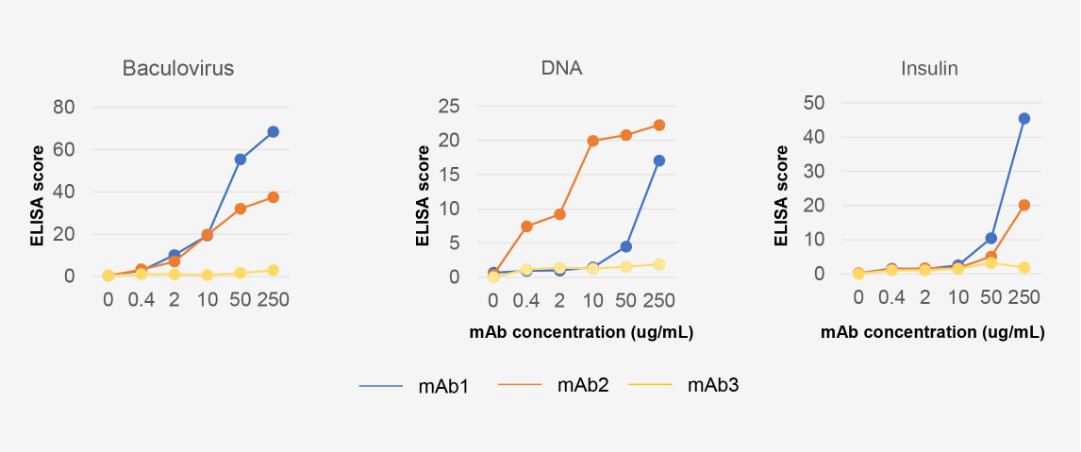
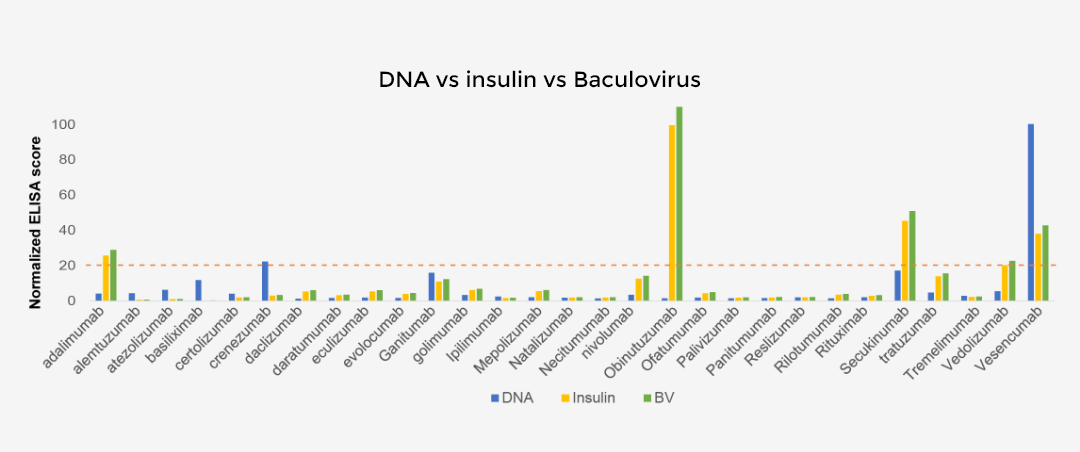
Baculovirus/DNA/Insulin ELISA
- Assessment of non-specific binding of mAbs
- Most sensitive assays to measure low-affinity charge-based interactions of mAbs
- High throughput, low cost, robust
Molecular Assessment Service Details:
(Micro-Developability)
Preliminary Stability Assessment Service Details:
(example stability protocol)
Stage III Molecular Assessment Service Details:
(Developability for Top Candidate)
LC-MS Services
- Detailed characterization of bispecific antibody by intact mass analysis
- High-throughput intact mass analysis for protein confirmation and quick glycan analysis (96-well plate)
- Detailed peptide mapping for sequence coverage, PTM, sequence variant analysis
- Disulfide mapping analysis to determine unknown disulfide linkage
- N-glycan analysis by LC-Fluorescence-MS/MS for complex glycosylation
Intact Mass LC-MS Service Details:
(For Protein Samples)
Peptide Map LC-MS Service Details:
(For Protein Samples)
Disulfide LC-MS Service Details:
(For Protein Samples)
N-glycan LC-MS Service Details:
(For Protein Samples)
LC-MS Based Protein Quantification Service Details:
Purity and Impurity Test
WuXi Biologics evaluates the physicochemical characteristics of proteins using a variety of analytical methods to elucidate the identity, structure and purity of the product.
Product related impurities including size variants can be evaluated by orthogonal analytical methods including CE-SDS and size exclusion chromatography (SEC) with both UV and multi-angle light scattering (MALS) detectors, while charge variants can be evaluated using ion exchange HPLC (IEX-HPLC) and imaged capillary isoelectric focusing (icIEF). Process-related impurities such as host cell protein, host DNA, and protein A can also be routinely tested to ensure contamination control. Finally, high-throughput endotoxin testing is also available for microbial control.
Routine Protein Production Analysis Service Details:
Protein Stability Service Details: